Getting therapeutic drugs past the blood-brain barrier has long been one of medicine’s most difficult challenges, limiting our ability to treat conditions like Alzheimer’s disease, Parkinson’s disease, and brain cancers. While manipulating gene expression in brain cells holds tremendous promise for treating these conditions, effectively delivering gene-targeting drugs to the brain has remained an elusive goal.
Against this backdrop, a research team from Tokyo University of Science (TUS), Japan, led by Professor Makiya Nishikawa, is exploring how to improve the delivery of antisense oligonucleotides (ASOs), a promising class of gene-targeting drugs, to the brain and other organs. With an emphasis on the United Nations’ sustainable development goals (SDGs), the team set out to improve good health and well-being (SDG 3) and promote industry, innovation, and infrastructure (SDG 9).
In their latest study, published online in the Journal of Controlled Release on February 18, 2025, the researchers delved into the mechanisms that govern how long these compounds remain in the bloodstream, what they bind to, and which tissues they can enter. This study was co-authored by Mr. Yukitake Yoshioka from TUS and Associate Director Syunsuke Yamamoto from Takeda Pharmaceutical Company Limited.
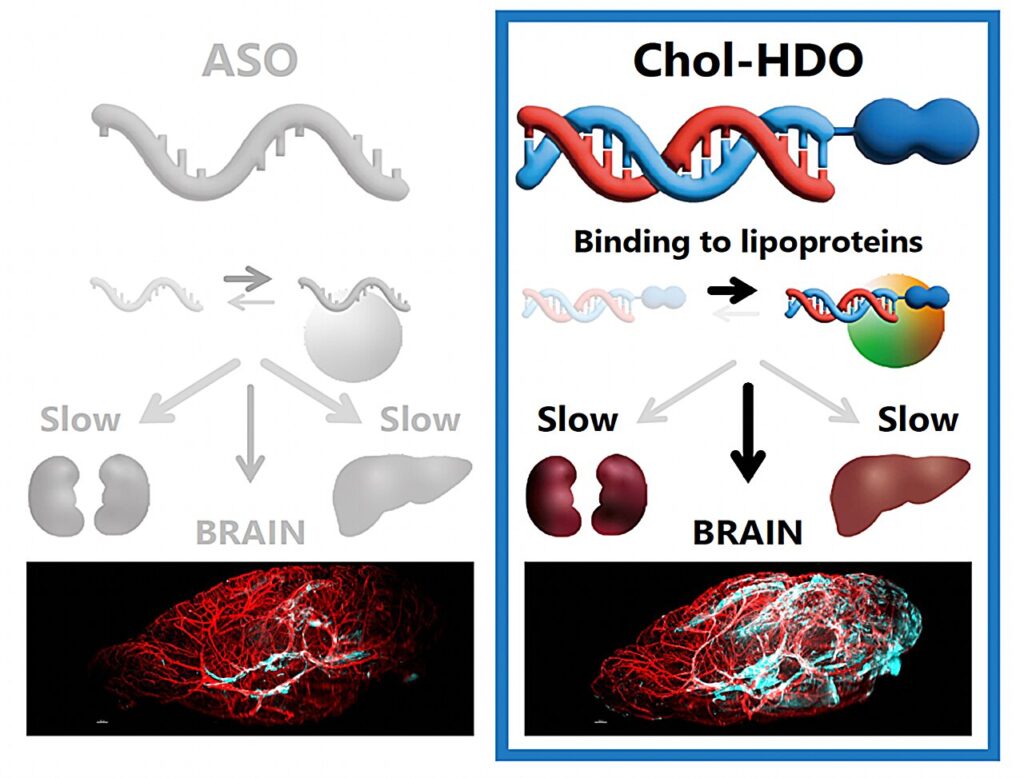
Given their ability to modulate genetic expression in cells, ASOs have become a hot topic in medical research. These compounds consist of a piece of single-stranded DNA with a base sequence that is complementary to a target messenger RNA (mRNA). By binding to their target, ASOs can prevent the production of specific proteins in cells. Despite their potential, ASOs fail to reach the brain effectively and tend to be quickly cleared from the bloodstream.
To address this issue, the researchers focused on a new type of gene-targeting compound called heteroduplex oligonucleotides (HDOs). HDOs operate similarly to ASOs but have an additional complementary RNA strand that enhances their stability and specificity.

Interestingly, this extra RNA strand can be further modified by attaching a cholesterol (Chol) molecule to create Chol-HDOs. Building on recent reports about the enhanced ability of Chol-HDO to reach various organs in the body—including the brain—Prof. Nishikawa’s team sought to clarify the pharmacokinetics of these compounds compared to ASOs and HDOs, shedding light on how they are distributed within the body.
To this end, the researchers conducted several experiments in rats and mice, using techniques such as liquid chromatography, tandem mass spectrometry, light-sheet fluorescence microscopy, and polyacrylamide gel electrophoresis. After detailed analysis, the team demonstrated that, unlike HDOs and ASOs, Chol-HDOs could penetrate the cerebral cortex beyond the blood vessels, which is an essential step toward potential treatments for brain diseases.
The key to this success lies in how Chol-HDOs interact with proteins in the blood. “We found that, while HDOs bind electrostatically to serum proteins with low binding affinity and are taken up by cells, Chol-HDOs bind tightly to serum proteins, including lipoproteins, via hydrophobic interactions,” explains Prof. Nishikawa. “This strong binding of Chol-HDOs to serum proteins results in slow clearance from the bloodstream.”
Interestingly, the researchers also showed that inhibiting scavenger receptors in cells reduces the uptake of both ASOs and Chol-HDOs in the liver and kidneys, shedding light on how these compounds are taken up by different organs.
Taken together, the findings of this study provide valuable insight into how brain-targeting drugs could be designed based on Chol-HDOs. “The possibility of efficiently delivering ASOs and other nucleic acid-based drugs to the brain may lead to the development of treatments for brain diseases with significant unmet medical needs,” remarks Prof. Nishikawa.
Today, over 55 million people are living with dementia, caused by diseases that could be treatable, or at least preventable, if we could deliver the right compounds beyond the blood-brain barrier. The same is true for brain cancers, with 300,000 cases reported annually worldwide. With continued research, modified HDOs could pave the way for a new generation of drugs that effectively target brain diseases, offering hope to millions of patients and their families around the world.