A team of chemists at the Indian Institute of Technology Madras, working with a pair of colleagues from the Jawaharlal Nehru Center for Advanced Scientific Research, both in India, has found that particles of minerals sometimes break down spontaneously when immersed in charged microdroplets, leading to the formation of nanoparticles.
In their study, published in the journal Science, the group conducted experiments with minerals and an electrospray device. R. Graham Cooks and Dylan T. Holden with Purdue University have published a Perspective piece in the same journal issue outlining the work.
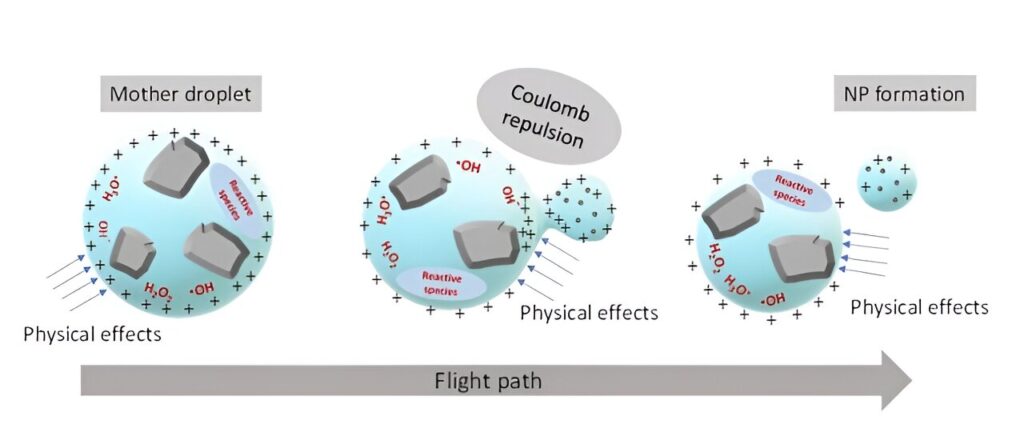
Prior research has shown that natural processes often result in the creation of nanoparticles and that many types of such nanoparticles exist in nature. But not much is known about how they are formed. In this new effort, the research team suspected that some of them may be the result of minerals becoming immersed in charged liquid particles. To find out if that might be the case, they designed an experiment to replicate such natural processes.
The researchers note that charged microdroplets are plentiful in the natural world, found in clouds and sea spray. To create their own charged microdroplets, they used an electrospray device.
When filled with water and electrically charged, it can produce a mist of charged droplets. In their experiments, the research team added mineral particles to the water before putting it in the spray device. They then captured samples of the charged microdroplets and other materials that were in the mist. They found many instances of nanoparticles being spontaneously expelled from the microdroplets into the air around them.
The researchers found that shortly after droplet formation, a double electric field was generated across its surface, producing a reactive sphere. That was followed by droplet fission when coulombic energy in the droplet exceeded its surface tension—and that was followed by expulsion of a mineral nanoparticle in the form of a microdroplet.