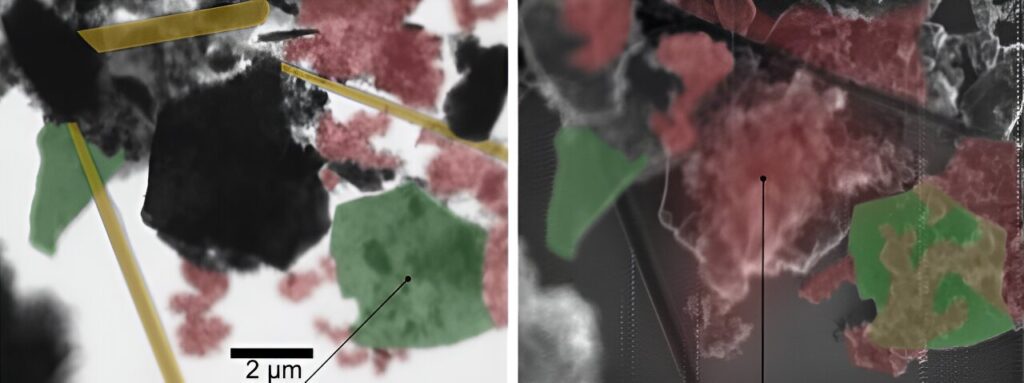
A new method in spectromicroscopy significantly improves the study of chemical reactions at the nanoscale, both on surfaces and inside layered materials. Scanning X-ray microscopy (SXM) at MAXYMUS beamline of BESSY II enables the investigation of chemical species adsorbed on the top layer (surface) or intercalated within the MXene electrode (bulk) with high chemical sensitivity.
The method was developed by a HZB team led by Dr. Tristan Petit. The scientists demonstrated, among others, first SXM on MXene flakes, a material used as electrodes in lithium-ion batteries. The paper is published in the journal Small Methods.
Since their discovery in 2011, MXenes have gathered significant scientific interest due to their versatile tunable properties and diverse applications, from energy storage to electromagnetic shielding. Researchers have been working to decipher the complex chemistry of MXenes at the nanoscale.
The team of Dr. Tristan Petit has now made significant progress in MXene characterization, as described in their recent publication. They utilized SXM to investigate the chemical bonding of Ti3C2Tx MXenes, with Tx denoting the terminations (Tx=O, OH, F, Cl), with high spatial and spectral resolution. The novelty of this work is to simultaneously combine two detection modes, transmission and electron yield, enabling different probing depths.
SXM provided detailed insights into the chemical composition and structure of MXenes. According to Faidra Amargianou, first author of the study, “Our findings shed light on the chemical bonding within MXene structure, and with surrounding species, offering new perspective for their utilization across various applications, especially in electrochemical energy storage.”
For the first time, SXM was employed to image MXenes, revealing details of the local bonding between titanium and terminations within the MXene structure. The researchers also examined the influence of different synthesis routes on MXene chemistry, shedding light on the impact of terminations on the electronic properties of MXene.
Furthermore, the application of SXM in analyzing MXene-based materials in lithium-ion batteries yielded valuable insights into changes in MXene chemistry after battery cycling. Amargianou explains, “The bulk of MXene electrode remains stable during electrochemical cycling with signs of possible Li+ intercalation. Electrolyte does not lead to degradation of the MXene and lays on top of the MXene electrode.”
In summary, this study provides valuable insights into the local chemistry of MXenes and underscores the potential of SXM in the characterization of other layered materials. Petit concludes, “This work highlights the significance of advanced chemical imaging techniques like SXM in unraveling the interactions of layered materials in complex systems. We are currently working on enabling in situ electrochemical SXM measurements directly in liquid environment. “