This article demonstrates how Raman spectroscopy is employed to investigate nanostructured materials for carbon capture. With climate change and increasing CO2 emissions, novel methods to reduce atmospheric CO2 concentrations are of great interest to governing bodies.
Image Credit: Renishaw plc – Spectroscopy
Carbon capture, utilization and storage (CCUS) could help to reduce carbon emissions to counter the negative effects of climate change. CCUS is vitally important in industries such as steel manufacture, chemical production and fossil fuel power generation to reach Net Zero carbon emissions.
Image Credit: Renishaw plc – Spectroscopy
Current CCUS technologies are varied in terms of maturity. For example, amine-based CO2 capture is used on a large scale during chemical production.1 More potential contenders are emerging that could perform better at lower unit costs but are still in the development phase.
Two novel materials with promising performance characteristics for carbon capture are discussed, including solid-amine nanoporous structures and graphene membrane filters.
Raman spectroscopy is a crucial part of developing and characterizing these cutting-edge materials. Renishaw’s range of spectrometers provides chemical and structural characterization, helping to achieve optimal synthesis of this advanced material.
Solid-Amine-Based Carbon Capture
Since 1930, amine scrubbing has been used as it allows high purity (>99%) CO2 collection.2
Amine scrubbing is a process that utilizes aqueous amine solutions to remove carbon dioxide (CO2) and hydrogen sulfide (H2S) from flue gases.
CO2 is usually captured using monoethanolamine solution (20–30 wt % in water) and then released at 100–120 °C temperatures. This process leaves vast amounts of degraded solvent as waste. The scrubbing technologies used today are uneconomical and energy-intensive as a result. This issue has renewed interest in solid-amine materials for carbon capture.
Carbon-based nanoporous structures have relatively high thermal stability and chemical resistance, making them a high choice for solid amine-based CO2 capture systems. Furthermore, they have the potential for large-scale production at low cost. A high percentage of porosity can be engineered to readily achieve large amounts of amine impregnation for effective CO2 absorption.
Professor Zheng-Xiao Guo and his co-workers at University College London, UK, reported a well-controlled and scalable method of obtaining carbon-based nanoporous impregnated with amine.
The group has synthesized highly hierarchical meso- and macro-porous graphene networks, prepared by thermal-shock exfoliation of graphene oxide (GO) at moderate temperatures of 300 ˚C for around five minutes.3 The degree of oxidation of the GO determines the strength of exfoliation to yield exceptional porosity: both the ultrahigh total pore volume (≥6 cm3 g−1) and the specific surface area (SSA, ≈800 m2 g−1) are significantly higher than for other mesoporous carbons, metal-organic frameworks (MOFs), zeolites and silicas. Raman analysis of the D- and G-bands of GO was performed on an inVia™ confocal Raman microscope to verify the differing degrees of chemical modification pre and post-exfoliation (Figure 1).
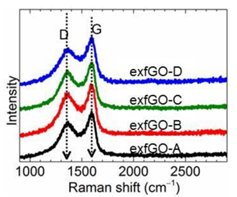
Figure 1. Raman spectra of the exfoliated graphene oxide (exfGO) samples represent a highly disordered state of the samples due to chemical modification. The exfGO samples are labeled A to D in order of increasing oxidation state. Note that the 2D band of graphene (≈2700 cm−1) is barely detected, indicating a loss of structure (order). (From: c7ta05789j1.pdf (rsc.org)).
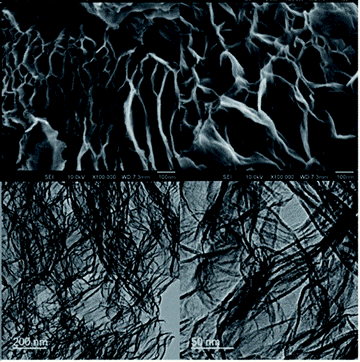
Figure 2. Highly networked surface morphology and porosity of exfGO-D samples determined by electron microscopy. The upper and lower rows show the scanning electron microscope (SEM) and tunneling electron microscope (TEM) micrographs, respectively. (From: Design of hyperporous graphene networks and their application in solid-amine based carbon capture systems – Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/C7TA05789J)).
The exfoliated GO samples were impregnated with triethylenetetramine (TETA). Testing the samples in a simulated flue-gas stream (75C, 100 ml min-1, 15% of CO2 in N2, bubbled through water) revealed a stable working CO2 capture capacity of >25 wt% (7.0 mmol g-1). These values are the best of any literature values for any type of solid-amine or porous solid carbon capture system.3
An Efficient and Cost-Effective Graphene Membrane Filter
Membrane filters make an attractive energy-efficient solution for carbon capture. The task of gas-sieving through nanoporous single-layer graphene to achieve molecular separation has seen over a decade of research.4
Professor Kumar Varoon Agrawal and his chemical engineers at Ecole Polytechnique Federale de Lausanne (EPFL) have recently developed a highly selective graphene filter membrane for carbon capture. This graphene filter is more efficient than existing commercial carbon capture technologies at a much lower expense of $30 per ton of carbon dioxide.5
The highly controlled synthesis method developed by Prof Agrawal’s group can achieve a narrow pore-size distribution (PSD) across graphene monolayers and a high pore density. This corresponds to sub-angstrom resolution for molecular differentiation between molecules of similar size: CO2/CH4; CO2/N2; O2/N2.
The resulting graphene membranes show excellent sieving performance with large O2 and CO2 permeance. The membranes are, therefore, an energy-efficient and scalable material for carbon capture.
Raman spectroscopy was employed to characterize the nanoporous single-layer graphene (N-SLG) films, which were synthesized by treatment with O3 in a reactor.6
The high sensitivity and fast mapping capabilities of Renishaw’s inVia confocal Raman microscope enabled the structural characterization of the nanoporous single-layer graphene films.
Raman analysis revealed ID/ID’ ratios below 3, indicating that the defects were mostly edge-like defects of graphite (Figure 3). The ID/IG ratio was measured using Fast Raman imaging, indicating that the porous defects were uniformly generated over a large area.
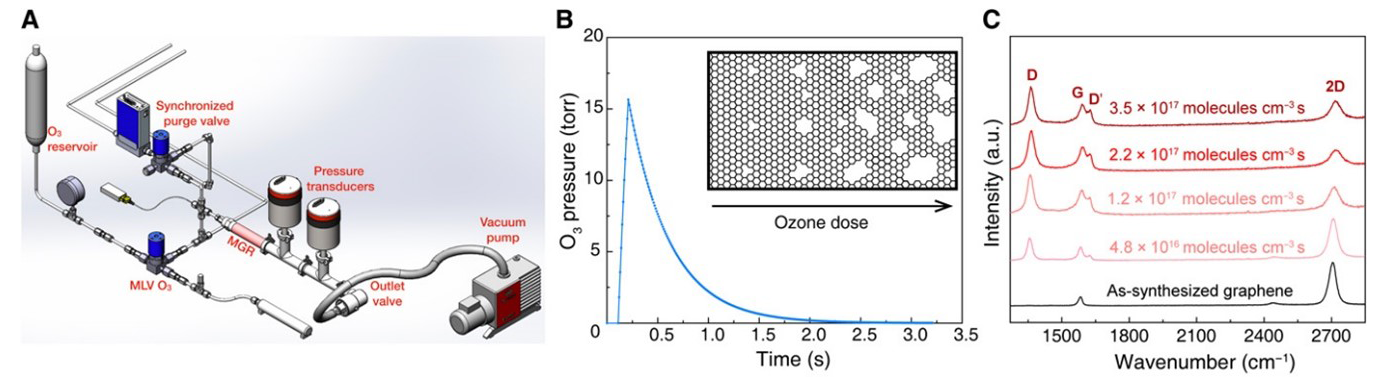
Figure 3. Precise incorporation of a high density of vacancy defects in nanoporous single layer graphene by millisecond gasification with O3. (A) Schematic of the reactor setup. (B) Profile of the O3 pulse in the reactor. (C) Raman spectroscopy analysis showing the evolution of the N-SLG with increasing O3 dose. Image Credit: Renishaw plc – Spectroscopy
New Materials Can Help to Decarbonize Industrial Processes
New functional materials are urgently being developed because of the demand to decarbonize industrial processes and fossil fuel power generation.
Researchers can use Raman spectroscopy to optimize the structure and chemistry of nanostructured materials. Numerous technologies which show great potential are being developed for carbon capture applications, giving exciting new opportunities for Raman analysis to be used to help meet these new demands.
References and Further Reading
- CCUS technology innovation CCUS in Clean Energy Transitions Analysis – IEA
- Review on CO2 Capture Using Amine-Functionalized Materials | ACS Omega
- Design of hyperporous graphene networks and their application in solid-amine based carbon capture systems – Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/C7TA05789J
- Selective molecular sieving through porous graphene | Nature Nanotechnology
- Graphene filter makes carbon capture more efficient and cheaper (phys.org)
- Millisecond lattice gasification for high-density CO2– and O2-sieving nanopores in single-layer graphene | Science Advances
This information has been sourced, reviewed and adapted from materials provided by Renishaw plc – Spectroscopy.
For more information on this source, please visit Renishaw plc – Spectroscopy.



