Inexpensive and readily available copper-based catalysts are considered ideal for the electrochemical CO2 reduction reaction (CO2RR) to produce multi-carbon products. The presence of copper oxides is crucial for generating high-value-added products in CO2RR.
However, the inevitable side hydrogen evolution reaction and the easy self-reduction reaction of copper oxide under the negative potentials diminish the catalytic activity and selectivity of CO2RR. Currently, designing a stable phase with both resistance to electrochemical self-reduction and high CO2RR activity is challenging.
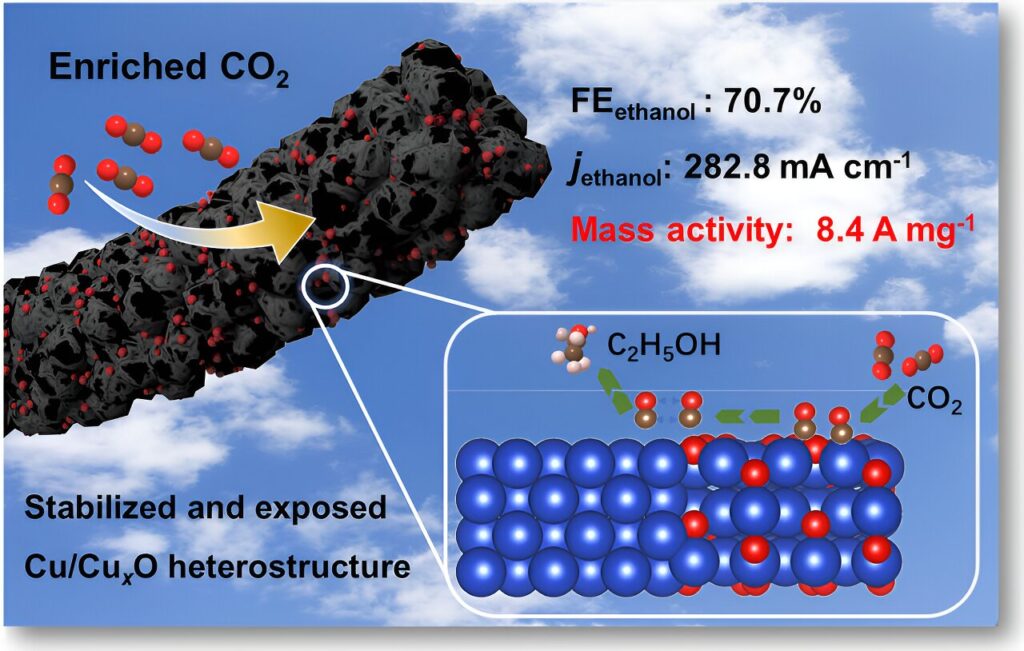
Recently, a research team led by Prof. Chuanxin He from Shenzhen University, China, sought to fully utilize the confinement effect and carrier effect of porous carbon nanofiber substrates on metal nanoparticles, significantly enhancing the exposure of active sites Cu/CuxO heterojunctions at the catalytic reaction interface.
The catalyst could maintain the structural stability of copper oxides under a current density of 400 mA cm‒2 and achieve an excellent CO2RR performance to ethanol with a Faradaic efficiency as high as 70.7% and a mass activity of 8.4 A mg‒1.
In this research, highly-dispersed copper nanoparticles within carbon nanofiber were firstly prepared via electrospinning, then the O2-plasma treatment was introduced to simultaneously create Cu/CuxO heterostructure and opening mesopores throughout those carbon nanofibers.
Specifically, the opening mesopores throughout carbon nanofibers can fully expose the Cu/CuxO sites to the three-phase interface compared with untreated carbon nanofibers, leading to high and stable catalytic activity with low metal loading amount.
Combined with the physical characterizations and in-situ spectral characterizations such as infrared and Raman spectroscopy analysis, a dynamic stabilized state of CuxO and the key signals of *CO and C–C bond are observed during the CO2RR process. Additionally, DFT calculations show that the presence of CuxO promotes the spillover of *CO intermediate to the Cu/CuxO interface, which can decrease the C–C coupling energy barrier to form C2H5OH during the CO2RR process.
The carbon substrate can enhance electron transport and act as an electron donor to neutralize the reduction of CuxO under a negative potential, which assists the stability of Cu/CuxO heterostructure and maintains 213-h stability at high current densities. The results were published in Chinese Journal of Catalysis.