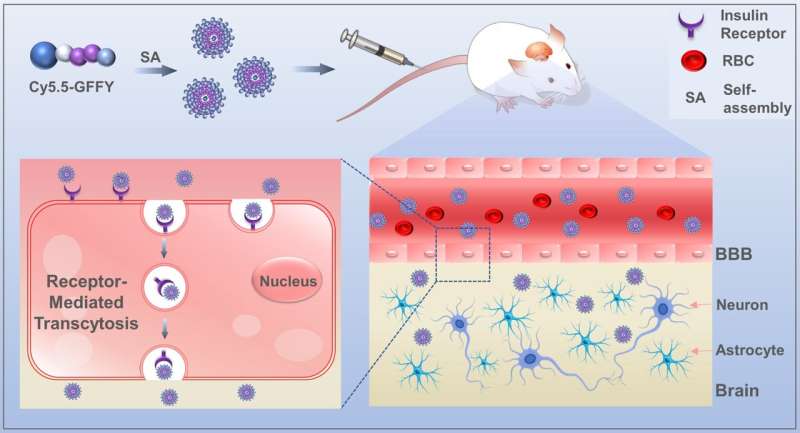
In a paper published in Science China Materials, a research team reports on a novel tetrapeptide, GFFY, which is capable of efficiently crossing the blood-brain barrier (BBB). The tetrapeptide is highly versatile in covalent labeling with Cy5.5 and the resulting Cy5.5-GFFY can self-assemble into nanospheres.
The researchers demonstrate that the nanospheres can efficiently deliver Cy5.5 to the brain by a fold increase of approximate 2.43, which is comparable to Cy5 penetration of damaged BBB in traumatic brain injury (about 3.5-fold of increase). Amino acid sequence changes would reduce the efficiency of self-assembling peptides in shuttling through the BBB.
Notably, the tetrapeptides tended to self-assemble to form nanomaterials when conjugated with hydrophobic molecules such as imaging agents Cy7 and ICG, which is beneficial for the development of nanomaterial-based BBB crossing strategies.
They also investigated the mechanism of efficient penetration of the BBB in vitro and in vivo by microscale thermophoresis and receptor-specific inhibitors, respectively, whereby the nanospheres specifically bind to the IR overexpressed on the BBB and then cross the BBB by IR-mediated transcytosis. The use of specific receptor inhibitors reduces the efficiency of self-assembled peptides to cross the BBB at both the cellular and animal levels.
Furthermore, the team’s Evans Blue (EB) results showed that no EB leakage was observed in the brains of any of the mice injected intravenously with Cy5.5-GFFY. Serum biochemistry and hematologic assays also showed similar primary assay values to controls. These results suggest that Cy5.5-GFFY has a favorable biosafety profile. It will provide a new approach to construct efficient brain-targeted delivery systems for diagnosis and treatments of diseases related to the central nervous system.