Flow-through reactors packed with enzymes can produce certain chemicals in a gentle and careful way. However, their performance has so far been limited. A research team from the Helmholtz-Zentrum Hereon and RWTH Aachen University has now been able to increase the yield a thousandfold.
Using a tailor-made nanomembrane, they have succeeded in bringing the molecules to be converted into much closer contact with the enzymes, thereby dramatically increasing the reaction rate. The new process could be used for the sustainable production of phosphate, among other things. The working group presents its results in the journal Nature Communications.
Enzymes are biocatalysts that can be used to produce chemicals in an environmentally friendly and energy-saving way. However, the process does not always make it easy to use them efficiently. One of the concepts is flow-through reactors. They consist of small channels to whose walls the enzymes adhere. When a solution flows through these channels, the molecules contained in the solution can dock onto the biocatalysts in order to react with their help to form the desired product.
So far, these reactors have not worked optimally, as they usually have millimeter-sized channels—the enzymes, on the other hand, are nanometer-sized. As a result, many of the molecules flowing through do not even come into contact with the biocatalysts and therefore have no opportunity for a chemical reaction.
Shock absorber for enzymes
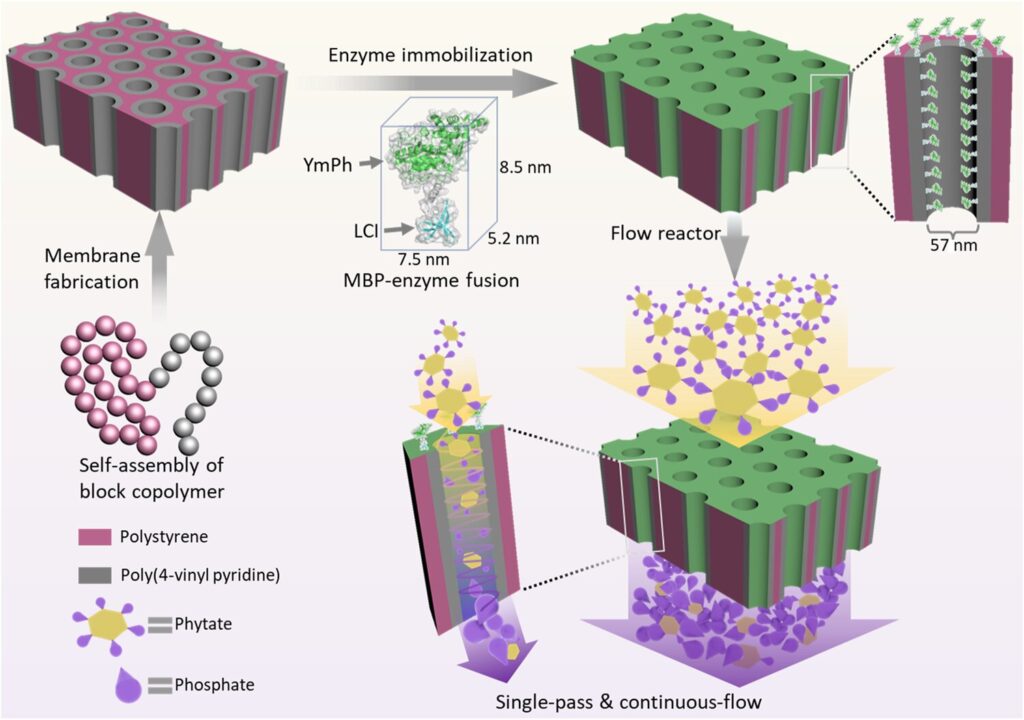
To solve this problem, the working group used a special membrane developed at the Helmholtz-Zentrum Hereon in Geesthacht. “This membrane is created by the self-assembly of so-called block copolymers,” explains Dr. Volker Abetz, Head of the Hereon Institute of Membrane Research and Professor of Physical Chemistry at the University of Hamburg. “Their surface has a high density of equally sized cylindrical pores.” These are tiny, with a diameter of just 50 nanometers. Below the surface is a more open porous structure made of the same block copolymer.
The scientists used a specially designed helper molecule—a kind of adhesive peptide—to lace these pore walls with enzymes. “It binds to the pore wall with one side and to the enzyme with the other,” explains Dr. Ulrich Schwaneberg, Professor of Biotechnology at RWTH Aachen University and member of the scientific management of the Leibniz Institute for Interactive Materials. “The peptide acts as a kind of shock absorber that keeps the enzyme at a certain distance from the pore wall at all times.”
The team used an enzyme called phytase for their prototype. It causes the decomposition of phytate, a phosphorus-containing compound that is found in cereals, among other things. In practice, the phytase enzyme is added to animal feed, for example. This promotes the release of biogenic phosphate, which can then be used as a sustainable fertilizer.
Successful endurance test
“The prototype of our flow reactor has a relatively simple design,” says Hereon researcher Dr. Zhenzhen Zhang. “The membrane is about the size of a sheet of paper, plus a system that allows the phytate solution to flow through the membrane.”
As a result, due to the narrow pores densely packed with enzymes, about a thousand times more phytate molecules could be converted into phosphate than in the previous flow-through reactors—a remarkable yield. It was also useful that the membrane pores were electrically positively charged and the phytate molecules negatively charged. The resulting attractive forces also helped to bring the molecules into contact with the enzymes.
“We tested the membrane for 30 days, and it lost very little of its efficiency,” says Zhang. “It should certainly be possible to scale up our reactor to an industrial scale.” As the Hereon process can also be used to produce membranes with smaller or larger pores, it should also be possible to equip the reactor with other enzymes that can then accelerate other chemical reactions.
However, there are still unanswered questions to be clarified. “We have not yet understood in detail how the membrane structures are formed,” explains Abetz. “If we succeed, we hope to be able to produce the cylindrical pores in the membrane in a much more targeted way than before.”