The conductance of classical electric components typically decays with increasing length. In general, this is also the same behavior found at the nanoscale with 1D molecular wires. Now, researchers have demonstrated that, once more, things are different in the nanoworld (i.e. there is plenty of room at the bottom).
Researchers at IMDEA Nanociencia and Oxford University have measured the conductance of porphyrin nanoribbons, obtaining extraordinary conductance properties—near perfect transmission—when the molecular energy level is in resonance with the electrode Fermi level. The work has been published in the Journal of the American Chemical Society.
The search for long molecular wires that can transport charge efficiently drives the field of molecular electronics. The problem since the beginning, however, has been that the conductance of molecular wires typically decays significantly with their length.
The reason behind this is an often strong mismatch between the energy of the transporting molecular orbitals and the electrode Fermi level (the highest occupied electronic state of a metal, where electron transport takes place). This mismatch means that electrons must tunnel through the molecular states, which results in an exponential decrease in the conductance as the length of the molecular wire is increased. This is typically assessed by building longer and longer compounds (i.e. adding successive units to an oligomer chain) and observing how the conductance changes.
As π-conjugated molecular compounds (i.e. compounds with alternating single-double or single-triple bonds) become longer, the gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) narrows, which should favor conductance.
In reality, the greater distance the electrons must tunnel wins out, and the conductance quickly becomes vanishingly small. The result is that molecules longer than about 3–4 nanometers normally become too resistive for single-molecule measurements. The inefficiency with which molecular junctions transport charge is a major factor hindering the development of electronic circuits based on molecules.
In their latest work, researchers jointly led by Dr. Edmund Leary aimed to create long, conductive, molecular junctions with low contact resistance to the electrodes. They selected porphyrin oligomers—polymer chains comprising a small number of repeat units—as the best candidates for molecular wires because of their stability at room temperature, rigidity and the fact they can be fused into tapes analogous to graphene nanoribbons. Furthermore, porphyrins are biological molecules, ubiquitous in nature (blood, plant leaves, enzymes, etc.).
An intriguing feature of porphyrins is that their properties strongly depend not only on the structure and length of the molecule, but on the way individual rings are connected. They can become either very resistive or very conductive wires depending on the bonds between neighboring rings, even though they are essentially composed of the same kind of atoms.
Dr. Leary and his team studied chains of porphyrin rings triply-fused along the length of the wire, which were designed and synthesized by the team at Oxford University led by Prof. Harry Anderson. These bonds allow highly efficient delocalization of electrons, a key feature for increasing the conductance of a molecule. They have extremely small HOMO–LUMO energy gaps, less than 1 eV for the longest compounds.
In their experiments, the researchers in the Leary group “fished” the molecules with the tip of a scanning tunneling microscope (STM) under ambient conditions. In this method, known as the STM breakjunction technique, the molecules are deposited onto a gold surface and a voltage is applied between the STM’s tip and the surface.
Using this “fishing” approach they catch single molecules and form and break on the order of hundreds–thousands of molecular junctions. The researchers measured the conductance as the electrodes are separated with a molecule wire in-between, which allowed them to be sure they have trapped just a single molecule. They also measured the length of the molecular junctions, providing a good check that they were really measuring the end-to-end properties of the wires.
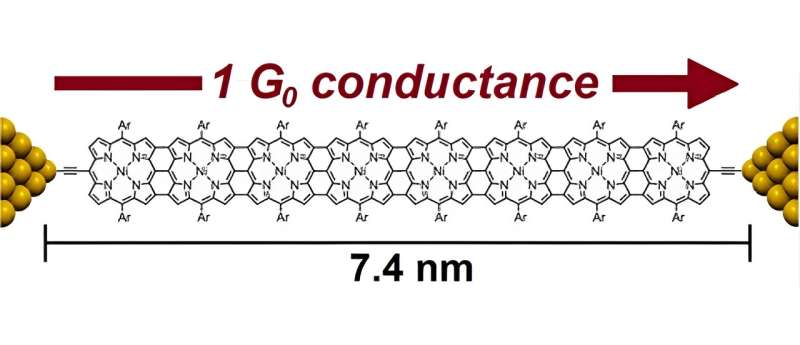
To their amazement, the conductance of the longest compound (> 7nm) was almost identical to the shortest compound, the monomer, which measures little more than 1 nm in length. This is only possible in the quantum regime, and shows that the HOMO–LUMO gap reduction compensates for the length increase even at such large distances.
However, measurements showed that electron transport is still a tunneling process at low-bias, and the conductance was still 100–1,000 times lower than theoretically possible.
Things started getting very interesting when the researchers applied a varying bias voltage to the junctions. In some of the junctions, impressively they found a maximum conductance at zero-bias, which decreased towards larger voltages. This is the reverse of the typical behavior.
Equally amazingly, the conductance in these junctions was much higher than previously observed and, in a significant number of junctions, reached the theoretical conductance limit of 77.5 μS, which is also known as 1 G0, the largest conductance possible through a single quantum channel. To put this into context, this is the typical conductance of individual atoms such as gold or silver.
Ballistic electron transport is known in metallic carbon nanotubes, and has also been claimed for very small molecules. The key aspect here is that this is the first time ballistic conductance has been observed at low-bias in long (> 7 nm) atomically-precise molecules with known atomic contacts connecting the wire to the electrodes. The measurements were done in air, and at room temperature. This is a true milestone for the field.
Among the possible mechanisms that could cause a maximum in conductance at zero-bias, Kondo is an obvious candidate. This was, however, immediately ruled-out as it is a purely low temperature process, occurring at few degrees Kelvin. At room temperature the only explanation for their results was perfect energy level alignment and ballistic conductance.
The trick to making the molecules conduct in this impressive manner involves changing the number of electrons on the molecule, converting them from neutral to charged molecules (doping). This happens when a sweeping bias voltage is applied to molecular junctions.
If a high-enough bias is reached, the molecular levels are brought into resonance with the metallic electrodes. This means that the molecular levels (either HOMO or LUMO) have the same energy as the electrons at the Fermi level in one of the electrodes.
In this resonant regime, electrons freely travel through the molecular wire, but occasionally one may become localized on the molecule. When this happens, a remarkable effect appears. Rather than the charge dissipating back onto the electrodes when the bias voltage is lowered back to zero, it frequently remains on the molecule for long periods, at least as long as the lifetime of the molecular junction.
Crucially, this changes the alignment of the molecular levels due to the charge imbalance on the molecule. This is the key aspect of the entire process. What the researchers believe is that the HOMO or the LUMO shift such that when the bias is brought back to zero, instead of having the original energy mismatch, a molecular energy level now aligns perfectly with the metallic Fermi level. This explains the low-bias ballistic conductance.
The most exciting moment for Edmund was to see the conductance peak at zero voltage. “We were expecting to see high conductances at high voltages, but not values at or around G0 at zero bias” Edmund explains.
“In fact, we were a little disappointed with the initial low-bias results, which showed that despite the ultra-narrow HOMO–LUMO gaps, electron transport is still suppressed for the neutral molecules. We knew we were on to something, however, when we started sweeping the bias voltage and started observing charged molecular junctions with ultra-high conductances. When we looked in detail at the data and found the conductance peaking at zero-bias, we realized that this was very good evidence for ballistic electron transport.”
The results show how molecules can behave like metal chains and conduct electricity at the theoretical limit, opening the exciting possibility of moving beyond 10 nm in single-molecule conductance experiments.

