Scientists at EPFL have developed a game-changing technique that uses light to manipulate and identify individual bacteriophages without the need for chemical labels or bioreceptors, potentially accelerating and revolutionizing phage-based therapies that can treat antibiotic-resistant bacterial infections.
With antibiotic resistance looming as a formidable threat to our health, scientists are on a constant quest for alternative ways to treat bacterial infections. As more and more bacterial strains outsmart drugs we have been relying on for decades, a possible alternative solution may be found in bacteriophages, which are viruses that prey on bacteria.
Phage therapy, the use of bacteriophages to combat bacterial infections, is gaining attraction as a viable alternative to traditional antibiotics. But there is a catch: Finding the right phage for a given infection is like searching for a needle in a haystack, while current methods involve cumbersome culturing, time-consuming assays.
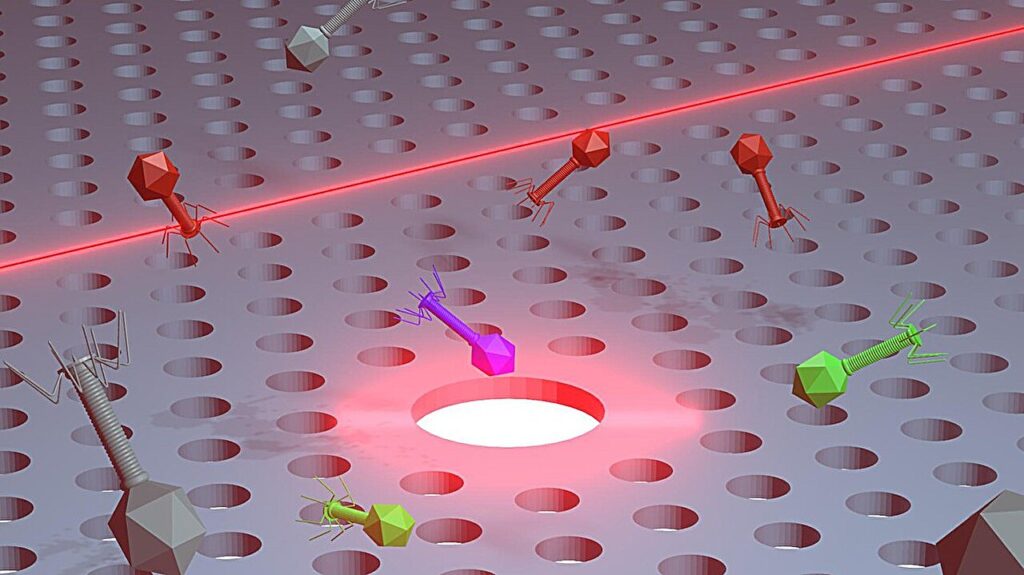
Now, scientists at EPFL, in collaboration with the CEA Grenoble and the Lausanne University Hospital (CHUV) have developed on-chip nanotweezers that can trap and manipulate individual bacteria and virions (the infectious form of a virus) using a minimal amount of optical power. The study, led by Nicolas Villa and Enrico Tartari in the group of Romuald Houdré at EPFL, is published in the journal Small.
The nanotweezers are a type of optical tweezers, scientific instruments that use a highly focused laser beam to hold and manipulate microscopic (e.g., virions) and even sub-microscopic objects like atoms in three dimensions. The light creates a gradient force that attracts the particles towards a high-intensity focal point, effectively holding them in place without physical contact.
Optical tweezers were first invented in 1986 by the physicist Arthur Ashkin who worked out the principles behind them in the late 1960’s. Ashkin’s technological innovation won him the 2018 Nobel Prize in Physics, and optical tweezers remain an intense field of research.
There are different types of optical tweezers. For example, free-space optical tweezers can manipulate an object in an open environment such as air or liquid without any physical barriers or structures guiding the light. But in this study, the researchers built nanotweezers embedded in an optofluidic device that integrates optical and fluidic technologies on a single chip.
The chip contains silicon-based photonic crystal cavities—the nanotweezers, which are essentially tiny traps that gently nudge the phages into position using a light-generated force field. The system allowed the researchers to precisely control single bacteria and single virions and acquire information about the trapped microorganisms in real time.
What sets this approach apart is that it can distinguish between different types of phages without using any chemical labels or surface bioreceptors, which can be time-consuming and sometimes ineffective. Instead, the nanotweezers distinguish between phages by reading the unique changes each particle causes in the light’s properties. The label-free method can significantly accelerate the selection of therapeutic phages, promising faster turnaround for potential phage-based treatments.
The research also has implications beyond phage therapy. Being able to manipulate and study single virions in real time opens up new avenues in microbiological research, offering scientists a powerful tool for rapid testing and experimentation. This could lead to a deeper understanding of viruses and their interactions with hosts, which is invaluable in the ongoing battle against infectious diseases.