Cancer stem cells (CSCs) are a rare population of cells in tumor tissues that drive tumorigenesis, recurrence, and metastasis. Therefore, the development of anti-tumor therapies that can eliminate CSCs has significant implications for cancer treatment. Photodynamic therapy (PDT) is a therapeutic modality that uses a specific laser wavelength to activate photosensitizers, generating large amounts of reactive oxygen species (ROS) to selectively inhibit tumor growth.
However, considering that oxygen is necessary for type II PDT and that hypoxia breeds CSCs, PDT is ineffective for those CSCs that are rooted in hypoxic tumor areas. Available strategies to overcome tumor hypoxia include using oxygen-carrying materials to deliver oxygen to the tumor, catalysis of hydrogen peroxide to oxygen at the tumor site, and inhibition of the intracellular oxygen consumption rate.
Nevertheless, the drawbacks associated with the above strategies have limited their clinical translation, including oxygen leakage from oxygen-carrying materials, insufficient endogenous hydrogen peroxide content, and complex preparation processes for multi-drug co-delivery systems.
Based on the above background, developing photosensitizers with a simple process capable of solving the problem of insufficient oxygen supply in the PDT process has a broad application prospect.
Prof. Zifu Li has been engaged in the development of nano drug delivery systems and small molecules for eliminating CSC. Since 2018.
Based on the strategy that unsaturated fatty acid modification can promote the self-assembly of hydrophobic structures, this work designed and synthesized oleic acid and cationic hemicyanine conjugate (CyOA). After co-precipitation, CyOA underwent self-assembly to form uniform and stable nanoparticles, realizing the construction of a single-molecule self-assembled nano drug delivery platform that did not need any excipients for stabilization.
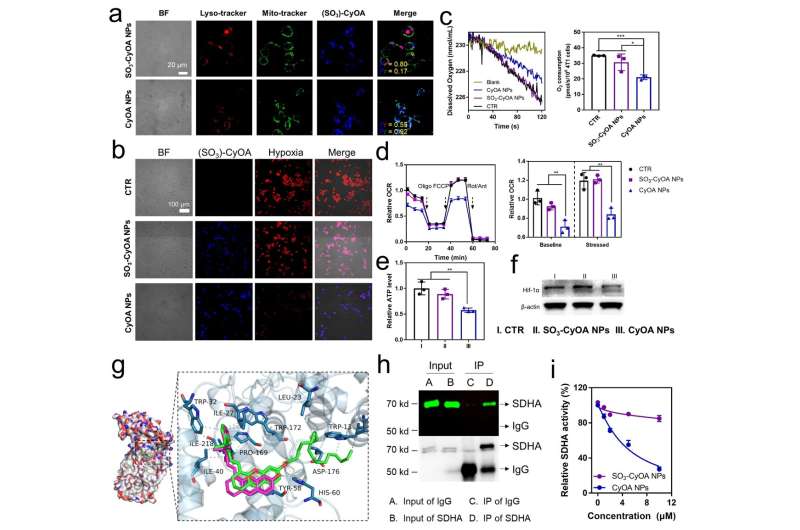
Since CyOA NPs are positively surface charged, they can efficiently accumulate in mitochondria in a membrane potential-dependent manner and undergo photochemical reactions in the presence of light, resulting in ROS bursts in mitochondria. Compared with SO3-CyOA NPs, CyOA NPs were 50.4-fold more phototoxic against breast cancer stem cells (BCSCs).
It is worth pointing out that this work is the first to find that cationic hemocyanine dyes can inhibit OXPHOS by targeting the mitochondrial complex II protein succinate dehydrogenase (SDHA).
Thus, CyOA NPs can act as both OXPHOS inhibitors and enable mitochondria-targeted PDT, addressing the inherent bottlenecks encountered with conventional PDT, including oxygen deprivation in solid tumors and the short lifespan and diffusion distance of ROS. In 4T1 and BCSC tumor models, CyOA NPs achieved higher tumor inhibition and less lung metastasis nodules compared to clinically used photosensitizer Hiporfin and exhibited no indication of toxicity in vivo.
This work developed a simple, efficient, and low-toxicity unimolecular self-assembled photosensitizer delivery system based on a commonly used hemicyanine. Compared to existing strategies related to solving the hypoxia problem faced by PDT for the treatment of solid tumors, the preparation process of CyOA NPs is simple and does not require any excipients for stabilization.
The results showed that CyOA is multifunctional, acting as both an OXPHOS inhibitor and photosensitizers, and enabling in vivo imaging. CyOA NPs address the inherent bottlenecks encountered by conventional PDT in the elimination of CSCs, including hypoxia in solid tumors, short lifetime and short diffusion distance of ROS. In summary, CyOA NPs have good prospects for clinical translation.
The findings are published in the journal Research.


