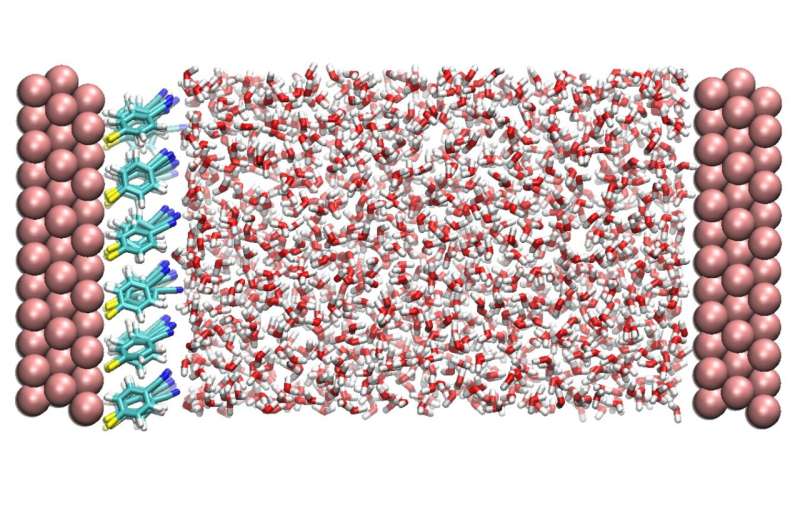
A collaborative team of experimental and computational physical chemists from South Korea and the United States has made an important discovery in the field of electrochemistry, shedding light on the movement of water molecules near metal electrodes.
This research holds profound implications for advancing next-generation batteries utilizing aqueous electrolytes.
In the nanoscale realm, chemists typically utilize laser light to illuminate molecules and measure spectroscopic properties to visualize molecules. However, studying the behavior of water molecules near metal electrodes proved challenging due to the overwhelming interference from metal atoms in the electrode itself.
Additionally, water molecules distant from the electrode surface also contribute to the response of the applied light, complicating the selective observation of molecules at the liquid-metal electrode interface.
Led by Professor Martin Zanni from the University of Wisconsin at Madison and Director CHO Minhaeng from the Center for Molecular Spectroscopy and Dynamics within the Institute for Basic Science (IBS) addressed this challenge with newly developed spectroscopic techniques coupled with computer simulations.
To minimize the interference from the metals, the authors coated the surface of the electrode with specially designed organic molecules. Then, surface-enhanced femtosecond (10-15 second) two-dimensional vibrational spectroscopy was employed to observe the changes in the movement of water molecules near the metal electrode.
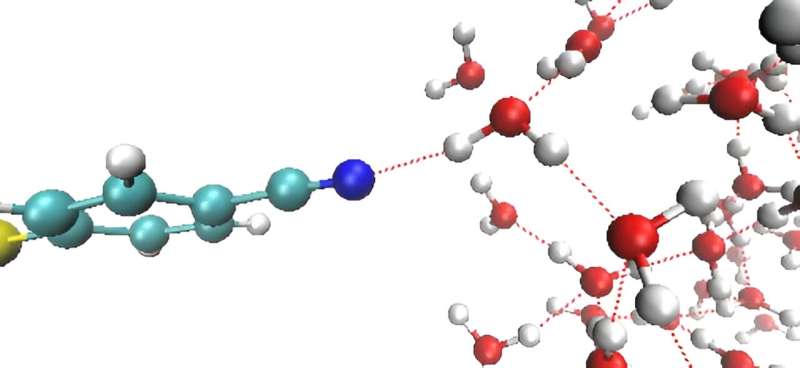
Depending on the magnitude and polarity of the applied voltage on the metal electrode, the researchers observed, for the first time, either deceleration or acceleration of the motion of water molecules near the electrode.
“When a positive voltage is applied to the electrode, the movement of nearby water molecules slows down. Conversely, when a negative voltage is applied, the opposite is observed both in femtosecond vibrational spectroscopy and in computer simulations,” explains Dr. Kwac.
“The results of this study provide crucial information for understanding electrochemical reactions, offering essential physical insights necessary for the research and development of aqueous electrolyte batteries in the future,” comments Director CHO Minhaeng of the IBS Center for Molecular Spectroscopy and Dynamics, a corresponding author of the study.
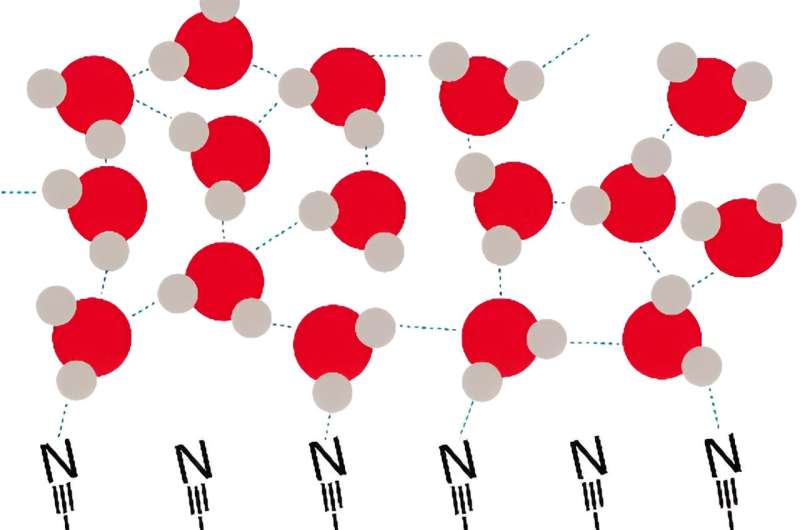
This outcome implies a close relationship between electrochemical reactions involving water on the surface of electrodes and the dynamics of interfacial water molecules. It is expected to not only advance our understanding of fundamental electrochemical processes but also pave the way for the design of more efficient and sustainable battery technologies.
This research was published in the Proceedings of the National Academy of Sciences.