Skoltech scientists have found a way to improve the most widely used technology for producing single-walled carbon nanotube films—a promising material for solar cells, LEDs, flexible and transparent electronics, smart textiles, medical imaging, toxic gas detectors, filtration systems, and more. By adding hydrogen gas along with carbon monoxide to the reaction chamber, the team managed to almost triple carbon nanotube yield compared with when other growth promoters are used, without compromising quality.
Until now, low yield has been the bottleneck limiting the potential of that manufacturing technology, otherwise known for high product quality. The study has been published in the Chemical Engineering Journal.
Although that is not how they’re really made, conceptually, nanotubes are a form of carbon where sheets of atoms in a honeycomb arrangement—known as graphene—are seamlessly rolled into hollow cylinders.
They vary in length, diameter, and so-called chirality (how the honeycomb pattern is “skewed”), as well as whether the tube is single-walled or has other, wider tubes around it, making it “multiwalled.” The properties of carbon nanotubes vary widely based on the above parameters. Chirality, for example, controls their electrical conductivity. Carbon nanotubes are manufactured as powder, thin films, fibers, and in other forms, depending on the application they are intended for.
Owing to their superb mechanical, electrical, optical, and thermal properties, carbon nanotubes are used in diverse products and technologies, from tear-resistant car tires and composite materials for wind turbine blades to flexible touchscreens and lithium-ion battery components.
The main applications of single-walled carbon nanotubes in the form of thin films are in electronic and optical devices, components, and solutions, particularly those intended to be flexible, stretchable, wearable, and transparent. Among them are lasers, light-emitting diodes and displays, solar cells, cables, transistors, mechanical, chemical, and light sensors, gas and liquid filtration systems, anti-static coatings, and even drug delivery vehicles.
The main technology for manufacturing single-walled carbon nanotube (SWCNT) films—and indeed most other forms of carbon nanotubes—is known as chemical vapor deposition (CVD) and encompasses several techniques that are variations on the same basic process.
Among such variations, floating catalyst (aerosol) CVD is used for the production of thin films, because it allows obtaining them in one step.
In this method, gaseous flows of carbon source (carbon feedstock for growing nanotubes, such as hydrocarbons, carbon monoxide, ethanol, etc.) and catalyst precursor (typically, precursor of iron nanoparticles—for example, ferrocene) are introduced into the high-temperature reactor.
The high temperature decomposes the precursor into catalytic nanoparticles followed by the decomposition of carbon source and deposition of carbon on their surface, the formation of fullerene hemisphere-like cap, and nanotube growth. At the outlet of the reactor, nanotubes are filtered simultaneously forming a “2D” network on the filter surface—the thin SWCNT film.
“The choice of carbon source depends on the desired properties of nanotubes. Carbon monoxide provides high product quality suitable for optics and electronics applications, but at the cost of a rather modest yield,” said study co-author Assistant Professor Dmitry Krasnikov of Skoltech.
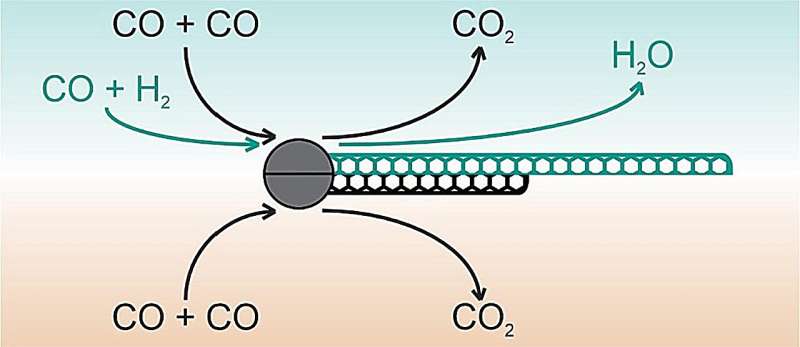
To solve this problem, researchers typically utilize growth promoters—additional compounds in the CVD reactor that increase nanotube growth or improve catalyst activation and/or lifetime. Typically, these are sulfur compounds, weak oxidants, such as carbon dioxide or water, or additional carbon sources. Nevertheless, all these options have their drawbacks.
“The current solutions could not significantly improve CO-based synthesis productivity. Two-, three-fold increase in the yield was typical for carbon dioxide, while sulfur addition was shown to be ineffective for the CO-based process,” commented Ilya Novikov, the main author of the publication who has recently defended his Ph.D. thesis devoted to nanotube synthesis at Skoltech.
“We considered hydrogen as a possible effective growth promoter. In previous works, it was found that its introduction into the CO atmosphere could trigger an extra reaction producing carbon in addition to the Boudouard reaction (the CO disproportionation: CO + CO → C + CO2)—CO hydrogenation (CO + H2 → C + H2O). We concluded it might work in our case too.”
After the thorough investigation of hydrogen’s effect on SWCNT synthesis yield as well as the properties of the nanotube product, the authors found a 15-fold increase in the synthesis productivity at 10 vol% concentration of H2 without deterioration of nanotube film structural properties and performance as a transparent conductor.
“Having studied the mechanisms involved in nanotube growth by optical spectroscopy and electron microscopy methods and performed a detailed study of the thermodynamics of the process, we concluded that carbon monoxide hydrogenation is indeed responsible for such a remarkable effect,” said Professor Albert Nasibulin, the head of the Laboratory of Nanomaterials at Skoltech.
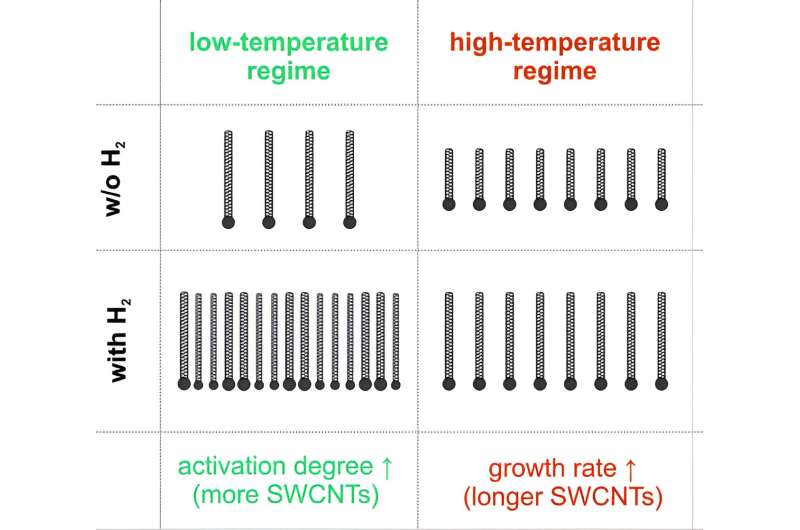
“Moreover, to explain its influence on the process in detail, we examined different temperature regimes for nanotube synthesis in addition to varying hydrogen concentration,” Krasnikov added.
“Unexpectedly, two different phenomena were observed: In the low-temperature regime, hydrogen significantly improves catalyst activation (the fraction of iron particles active for catalysis), thereby boosting the yield, while, in the high-temperature regime, it enhances nanotube growth, resulting in longer nanotubes with higher conductivity of the films.”
“Thus, we believe that this study solves two important problems at once. On the one hand, a considerable improvement in synthesis productivity significantly extends the applications of CO-based aerosol CVD processes and puts this method close to industry-level nanotube production. On the other hand, in this work, we have managed to discover fundamental mechanisms behind nanotube growth based on CO disproportionation, which should be extremely useful for a deeper understanding of nanotube CVD synthesis in general,” Nasibulin concluded.