There is an urgent need to address climate change, making the development of sustainable energy alternatives more important than ever. While proton-exchange membrane fuel cells (PEMFCs) have shown great promise for energy production, particularly in the transportation industry, there is a long-standing problem with their durability and cost.
A Western research team has addressed the issue with a new cobalt-modified nanomaterial making PEMFCs more robust, readily sourced and environmentally sustainable demonstrating just a two percent loss in efficiency rate following 20,000 cycles in a durability test.
The new nanomaterial is used to enhance oxygen reduction reaction (ORR), the process that forms water in the fuel cell allowing a higher current for more efficient power generation. The cobalt-modified nanomaterial also reduces the reliance on platinum to construct these fuel cells. A costly precious metal, and mined primarily in South Africa, only a few hundred tons of platinum are produced annually.
Tsun-Kong (T.K.) Sham, Xueliang (Andy) Sun, Ali Feizabadi and their collaborators at Western’s department of chemistry and Western Engineering introduced the new cobalt-modified ORR catalyst in The Journal of Physical Chemistry C.
“Cutting-edge approaches, including alloying platinum with other transition metals and crafting core-shell structures, present exciting possibilities by reducing the demand for platinum in fuel cells while maintaining exceptional catalytic activity,” said Sham, Canada Research Chair in Materials and Synchrotron Radiation and a senior author on the study. “But despite significant progress, the Achilles’ heel remains the durability of these catalysts due to inherently unstable structures.”
The Sham and Sun team used a method called ‘cobalt doping’ to modify the surface and near-surface areas of platinum-palladium core-shell nanoparticles.
“Doping is the practice of introducing very small amounts of particular foreign atoms into the crystalline structure of a nanoparticle to modify its electronic properties. These foreign atoms are referred to as dopants,” said Feizabadi, a former research analyst in the Sham research group and lead author of the study.
The new cobalt-doped nanoparticles demonstrate exceptional stability, enduring just a two percent loss in initial activity after a ‘gruelling’ 20,000 cycles of an accelerated durability test, used to gain deeper insights into the degradation mechanisms of catalysts in controlled laboratory settings.
“This underscores cobalt’s remarkable role in enhancing catalytic activity and reinforcing the catalyst’s structural integrity,” said Sham, a global leader in developing new X-ray spectroscopic techniques.
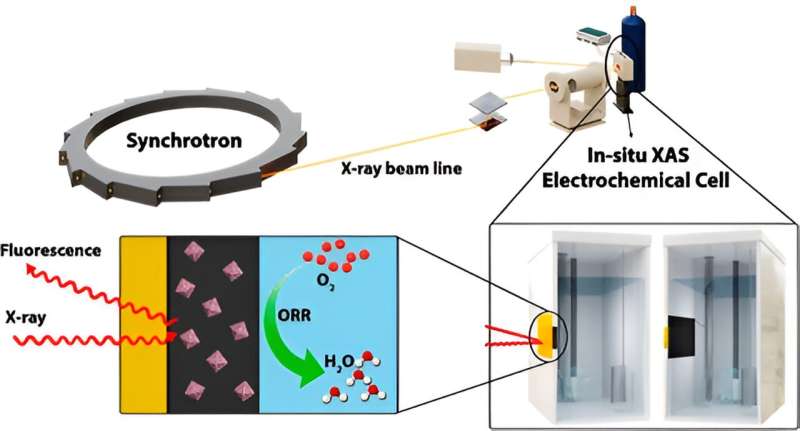
For a better understanding of catalyst behavior and composition, the research team studied the new nanoparticles using Hard X-ray Micro-Analysis Beamline at Canadian Light Source, Canada’s national synchrotron light source facility at the University of Saskatchewan.
The nanoparticles were also analyzed at the Advanced Photon Source in Lemont, Illinois and the Taiwan Photon Source for the study.
“These cobalt-doped nanoparticles hold immense promise as highly efficient and enduring ORR catalysts, representing a significant advancement in the realm of fuel cell technology,” said Sun, Western Engineering professor and leading expert in nanomaterials and clean energy.
“This comprehensive approach sheds new light on catalyst behavior and structure, bringing us one step closer to sustainable energy solutions.”