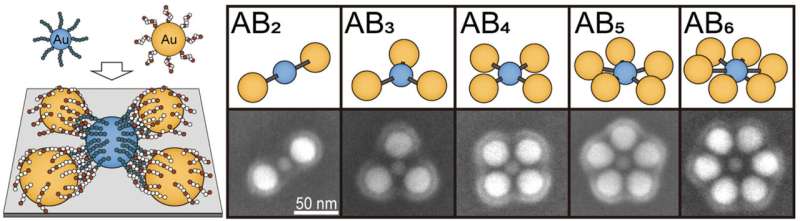
In the incredibly small world of molecules, the elementary building blocks—the atoms—join together in a very regular pattern. In contrast, in the macroscopic world with its larger particles, there is much greater disorder when particles connect.
A research team at the University of Göttingen has now succeeded in achieving the same precise arrangement of atoms shown in molecules, but using nanometer-sized particles, known as “plasmonic molecules”—combinations of nanoscale metallic structures that have unique properties. The results were published in Angewandte Chemie International Edition, which has classified the article as a “very important paper.”
There is a transition area between molecular and macroscopic levels, an in-between zone called the nanometer range, where there is often a disordered aggregation of particles. Precise arrangement of nanometer-sized structures is one of the major challenges in the ongoing miniaturization in electronics, optics and medicine.
In this novel process developed by Dr. Yinging Cai and Professor Philipp Vana at Göttingen University’s Institute of Physical Chemistry, the nanometer-sized particles are mixed together as in a chemical reaction and then arrange themselves completely independently into molecule-like structures.
“The approach here is to connect the particles using tailor-made polymer chains that interlock like two hands. We can control the force of this handshake via the type and length of the polymers and via the solvent used in which the reaction takes place,” explains Cai. The result is plasmonic molecules, which all have the same regular arrangement and can be produced quickly in large quantities—an important prerequisite for making these compounds useful and useable for a multitude of functions in the world of nanotechnology.
“By developing these plasmonic molecules, we have been able to establish chemical principles in the nanoworld that open up a whole new cosmos,” says Vana. “And we may just be witnessing the birth of a new kind of plasmonic chemistry that could lead to a wealth of new nanomaterials.”