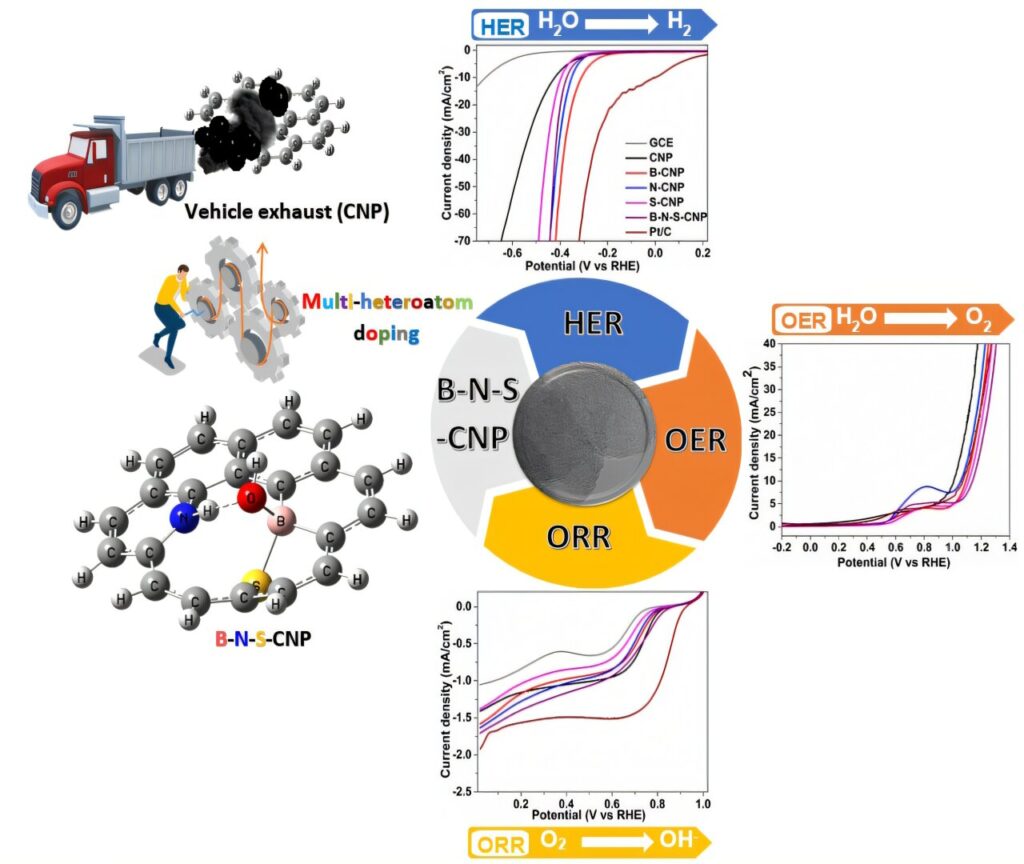
We have developed a breakthrough method to convert carbon nanoparticles (CNPs) from vehicular emissions into high-performance electrocatalysts. This innovation provides a sustainable approach to pollution management and energy production by repurposing harmful particulate matter into valuable materials for renewable energy applications.
Our work, published in Carbon Neutralization, addresses both environmental challenges and the growing demand for efficient, cost-effective clean energy solutions.
Advancing electrocatalysis with multiheteroatom-doped CNPs
By doping CNPs with boron, nitrogen, oxygen and sulfur, we have significantly enhanced their catalytic performance. These multiheteroatom-doped nanoparticles exhibit remarkable efficiency in key electrochemical reactions. Our catalysts demonstrate high activity in the oxygen reduction reaction (ORR), which is essential for fuel cells and energy storage systems, as well as in the hydrogen evolution reaction (HER), a crucial process for hydrogen fuel production.
Additionally, they show superior performance in the oxygen evolution reaction (OER), advancing water splitting for green hydrogen generation. By optimizing the composition of these materials, we have created an effective alternative to conventional precious metal-based catalysts, improving both cost-efficiency and sustainability.
Scientific insights and performance metrics
Using a combination of experimental analysis and density functional theory (DFT) modeling, we have gained deeper insights into the structural and electronic properties of these doped CNPs. Our boron-doped CNPs demonstrated an overpotential of 338 mV at 10 mA/cm², while B-N-S-CNPs exhibited a Tafel slope of 83.09 mV/dec, indicating superior reaction kinetics.
High-resolution TEM imaging revealed a sponge-like fractal structure, which enhances charge transfer and increases the number of active reaction sites. Raman spectroscopy confirmed increased disorder in heteroatom-doped CNPs, generating additional active sites for energy conversion.
Furthermore, modifications to the surface chemistry disrupted electroneutrality, improving adsorption and reaction efficiency and resulting in a more robust catalytic system. These material advancements allow us to reduce reliance on costly platinum-based catalysts, making clean energy technologies more viable and accessible.
Industrial applications and future prospects
Our research has far-reaching implications for clean energy and sustainable transportation industries. These catalysts can be integrated into fuel cells, enabling more efficient power generation for electric vehicles and energy storage systems. They also play a vital role in hydrogen production, supporting the transition to a hydrogen-based economy. Additionally, their use in renewable energy storage systems enhances the stability of wind and solar power generation.
While our findings demonstrate significant promise, further research is needed to scale up production, optimize material stability, and integrate these catalysts into commercial applications. Overcoming challenges related to large-scale synthesis and ensuring long-term durability will require collaboration between scientists, industries, and policymakers.
By refining manufacturing processes and developing sustainable extraction methods, we can transform pollution into a valuable energy resource, supporting the transition to a circular economy. With continued advancements, we can turn vehicular emissions from an environmental burden into a solution for clean and sustainable energy.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.