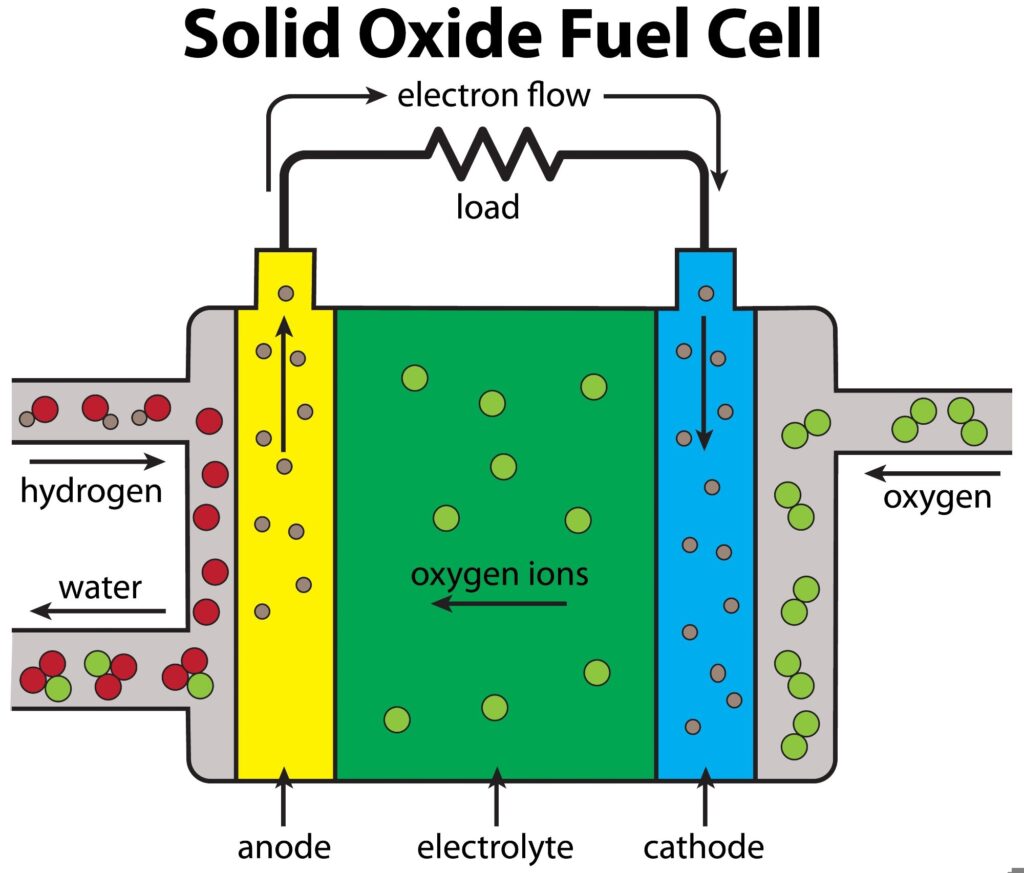
Solid oxide fuel cells (SOFCs) are electrochemical devices that use a solid ceramic electrolyte to conduct oxygen ions from the cathode to the anode, generating electricity through an electrochemical reaction. Operating at high temperatures, SOFCs enable the direct conversion of chemical energy from hydrogen or hydrocarbon fuels into electrical energy, offering fuel flexibility and low emissions.1
Image Credit: gstraub/Shutterstock.com
The ceramic electrolyte is central to this process, allowing ionic conductivity while remaining impervious to electrons, thereby ensuring efficient energy conversion. With the increasing demand for cleaner and more efficient energy solutions, advancements in SOFC technology—particularly in thin-film electrolyte membranes—are improving the performance and scalability of these systems, contributing to a more sustainable energy future.1-2
Principles of Thin-Film Electrolyte Membranes in SOFCs
The electrolyte membranes in SOFCs are essential components that conduct oxygen ions from the cathode to the anode, facilitating the electrochemical reaction that generates electricity.3
These membranes must maintain high ionic conductivity while preserving structural integrity under the extreme operating conditions of SOFCs, which involve temperatures ranging from 500 °C to 1,000 °C. They also act as barriers, preventing gas mixing between the anode and cathode, essential for maintaining cell efficiency and safety.2-3
Thin-film technology represents a significant advancement over traditional bulk electrolytes in SOFCs. By reducing the thickness of the electrolyte layer to the micrometer or sub-micrometer scale, thin-film membranes minimize ohmic resistance, enabling faster ion conduction and improving thermal efficiency.2
Additionally, using less material lowers overall costs and allows for lower operating temperatures, which enhances system longevity and reduces thermal stress on cell components. Advanced fabrication techniques, such as pulsed laser deposition (PLD) and chemical vapor deposition (CVD), provide precise control over the structure and composition of these thin films, optimizing their performance for modern energy applications.3-4
Recent Advances in Thin-Film Electrolyte Materials
Materials Development
Advances in material science have significantly improved thin-film electrolyte membranes for SOFCs. New ceria-based materials, such as gadolinium-doped ceria (GDC) and samarium-doped ceria (SDC), have gained attention due to their superior ionic conductivity and enhanced resistance to degradation at intermediate operating temperatures.5
These materials allow SOFCs to function efficiently at lower temperatures, reducing thermal stress on components and extending their operational lifespan. Additionally, proton-conducting materials like yttrium-doped barium zirconate (BZY) are being developed to further enhance ionic mobility and compatibility with various fuels, making them promising candidates for next-generation SOFCs.6
Fabrication Techniques
Cutting-edge fabrication methods have transformed the production of thin-film electrolyte membranes. PLD and CVD enable precise control over membrane thickness, structure, and composition, ensuring optimal ionic conductivity and mechanical stability.4
Sol-gel processes have also emerged as a cost-effective alternative, capable of producing uniform, defect-free films. These techniques allow for the fabrication of ultra-thin layers with improved microstructures, reducing material usage and minimizing defects, such as pinholes and pores, that could compromise cell performance.4
Performance Enhancements and Applications
The combination of advanced materials and fabrication techniques has led to performance improvements in SOFCs. Thin-film electrolyte membranes increase power densities by minimizing ohmic resistance and improving thermal efficiency. Their ability to operate at intermediate temperatures enhances durability by reducing material degradation and thermal cycling.3, 7
These innovations have allowed SOFCs to achieve longer operational lifespans and greater adaptability to various applications, such as portable power generation and integration into renewable energy systems. These advances highlight the potential of thin-film electrolyte membranes in driving the development of more efficient, durable, and cost-effective SOFC technologies.2,7
In clean energy contexts, SOFCs with thin-film membranes offer a sustainable solution for power generation, producing electricity with minimal greenhouse gas emissions. Hydrogen-fueled SOFCs, in particular, represent a key innovation, providing a pathway to eliminate CO2 emissions and offering an alternative to lithium-ion batteries, which pose significant recycling and disposal challenges.1, 8
Additionally, the flexibility of SOFCs to utilize various fuels, including hydrogen, methane, and syngas, reduces reliance on fossil fuels and supports environmental sustainability.7 These systems play a key role in the transition to renewable energy technologies and the development of zero-emission transportation and industrial applications.
Challenges and Future Directions
Scaling up the production of thin-film electrolyte membranes for SOFCs remains a critical challenge, as achieving both high performance and cost-effectiveness is technically demanding. Fabrication processes must produce large-scale cells with thin, gas-tight electrolytes (often less than 5 µm thick) while maintaining low grain boundary resistance and structural stability.1
Long-term stability is another concern, as materials must endure harsh real-world conditions, including thermal cycling, mechanical stresses, and potential chemical degradation during extended operation. The complexity of interfacial processes, such as gas/solid interactions, further complicates the optimization of these membranes.9
Traditional anode materials, such as Ni-based cermets, encounter issues like carbon deposition and degradation when operating with hydrocarbon fuels or at reduced temperatures, requiring external reforming or material modifications. Cathode materials also face challenges, with commonly used La1-xSrxMnO3−δ (LSM) and La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF)-based perovskites exhibiting performance degradation over time. This highlights the need for a better understanding of their failure mechanisms.1-2
The future of thin-film electrolyte membranes in SOFCs hinges on cost reduction and material innovation. Advancements in manufacturing methods and material development offer promising solutions to overcome current challenges. Techniques like PVD, CVD, and sol-gel processing enable precise control over thin-film properties, such as grain size, uniformity, and doping levels, enhancing the overall performance and stability of SOFCs.4, 9
These methods enable the fabrication of complex multilayer structures, optimizing the interaction between electrodes and electrolytes. Future material developments aim to improve established electrolyte compositions, such as GDC, and explore novel mixed-conducting ceramics to enhance ionic conductivity and reduce degradation at lower operating temperatures.9
Additionally, reducing the operating temperature of SOFCs below 600 °C could expand their applicability in areas dominated by low-temperature fuel cells, such as the automotive industry, while lowering material costs and improving thermochemical stability. Efforts to replace traditional noble metals and hazardous materials with cost-effective and environmentally friendly alternatives could further drive commercialization.1,4
By addressing these challenges, thin-film SOFCs can become a cornerstone technology for sustainable energy systems, offering scalable, efficient, and low-emission solutions for diverse applications.
References and Further Reading
1. Zhang, J.; Ricote, S.; Hendriksen, PV.; Chen, Y. (2022). Advanced Materials for Thin‐Film Solid Oxide Fuel Cells: Recent Progress and Challenges in Boosting the Device Performance at Low Temperatures. Advanced Functional Materials. https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202111205.
2. Choolaei, M.; Vostakola, MF.; Horri, BA. (2023). Recent Advances and Challenges in Thin-Film Fabrication Techniques for Low-Temperature Solid Oxide Fuel Cells. Crystals. https://www.mdpi.com/2073-4352/13/7/1008
3. Chasta, G.; Himanshu; Dhaka, MS. (2022). A Review on Materials, Advantages, and Challenges in Thin Film Based Solid Oxide Fuel Cells. International Journal of Energy Research. https://onlinelibrary.wiley.com/doi/full/10.1002/er.8238
4. Tang, M.; Niu, Y.; Muhammad, W.; Muhammad, S.; Zhong, Z.; Muhammad, S.; Pang, Y.; Wan, Z.; Chen, N.; Qiao, L. (2024). Advances in Solid Oxide Fuel Cell Electrolyte Fabrication by Pulsed Laser Deposition. International Journal of Hydrogen Energy. https://www.sciencedirect.com/science/article/pii/S0360319923045421
5. Das, S.; Bhaskar, R.; Narayanan, KB. (2024). Multifunctional Applications of Gadolinium-Doped Cerium Oxide (Ce1–Xgdxo2–∂) Ceramics: A Review. Journal of Rare Earths. https://www.sciencedirect.com/science/article/pii/S1002072123003575?via%3Dihub
6. Tariq, U.; Khan, MZ.; Gohar, O.; Babar, ZUD.; Ali, F.; Malik, RA.; Starostina, IA.; Rehman, J.; Hussain, I.; Saleem, M. (2024).Bridging the Gap between Fundamentals and Efficient Devices: Advances in Proton-Conducting Oxides for Low-Temperature Solid Oxide Fuel Cells. Journal of Power Sources. https://pubs.acs.org/doi/10.1021/acs.energyfuels.4c03683
7. Li, J.; Cheng, J.; Zhang, Y.; Chen, Z.; Nasr, M.; Farghali, M.; Rooney, DW.; Yap, PS. Osman, AI. (2024). Advancements in Solid Oxide Fuel Cell Technology: Bridging Performance Gaps for Enhanced Environmental Sustainability. Advanced Energy and Sustainability Research. https://scholar.xjtlu.edu.cn/en/publications/advancements-in-solid-oxide-fuel-cell-technology-bridging-perform
8. Kalinina, E.; Pikalova, E. (2021). Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology. Materials. https://www.mdpi.com/1996-1944/14/19/5584
9. Dhawale, DS.; Biswas, S.; Kaur, G.; Giddey, S. (2023). Challenges and Advancement in Direct Ammonia Solid Oxide Fuel Cells: A Review. Inorganic Chemistry Frontiers. https://pubs.rsc.org/en/content/articlelanding/2023/qi/d3qi01557b