Researchers at Nagoya University in Japan have addressed a significant challenge in nanosheet technology. Their innovative approach employs surfactants to produce amorphous nanosheets from various materials, including difficult-to-synthesize ultra-thin amorphous metal oxides such as aluminum and rhodium. This breakthrough, published in Nature Communications, sets the stage for future advances in the application of these nanosheets such as those used within fuel cells.
The upcoming generation of nanotechnology requires components that are just a few nanometers thick. These ultrathin layers, which are essential for improving functionality, are known as nanosheets.
However, their small size poses difficulties for catalytic reactions. Many of these sheets maintain a regular shape with minimal defects. But catalysis generally relies on these defects for its reactions.
Furthermore, their production is challenging due to the absence of layers, rendering traditional exfoliation techniques that depend on layering ineffective. This limitation has confined their production to typical materials, such as carbon and silica, rather than metal oxides and oxyhydroxides using materials like rhodium that are useful in technology.
To bridge this gap, a research group led by Assistant Professor Eisuke Yamamoto and Professor Minoru Osada at the Institute for Materials and Systems Research (IMaSS) at Nagoya University devised an adaptable synthesis method.
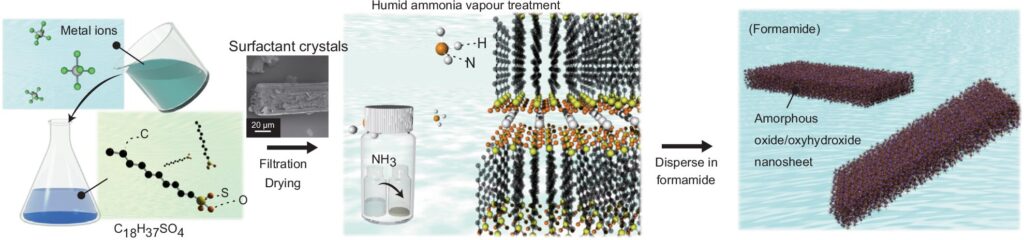
The process starts with a solid-state surfactant, which aids in arranging the metal ions within its framework, particularly in the areas between its layers, known as the interlayer space. Since amorphous nanosheets do not have layers, the surfactant layers serve as a substitute.
Osada is enthusiastic about the beauty of the process. “The surfactant crystals actually synthesized are beautiful under an optical microscope,” he says. “It is possible to confine a variety of metal ions in these surfactant crystals and create a variety of crystals.”
Water is then added, which interacts with the metal ions that have been arranged in the surfactant layers. It triggers a reaction known as hydrolysis that leads to the partial breakdown of these ions and the formation of small, isolated clusters.
The clusters can be arranged into an organized structure with the help of a solvent, specifically a chemical called formamide. This organization is directed by the initial crystal shapes of the surfactant through a process known as templating, where the metal clusters create sheets that replicate the shape of the surfactant crystals.
This method created amorphous nanosheets about 1.5 nm thick using gallium ions. Building on this success, Yamamoto and Osada applied the technique to synthesize others from challenging metal oxides and oxyhydroxides such as aluminum and rhodium.
“Amorphous nanosheets on this scale should have excellent catalytic activity, attributed to numerous defects resulting from their disordered structure,” explains Professor Osada. “These defects are excellent active sites for catalytic reactions. These amorphous sheets offer a vastly different functionality compared to traditional nanosheets.”
This innovative method not only synthesizes a variety of nanosheets with different metal species but also allows the combination of multiple metal types in one sheet, opening doors to new materials and properties.
“The new classes of materials synthesized through this technique are expected to drive advancements in the fields of two-dimensional and amorphous materials, potentially leading to novel physical properties and applications,” Osada says.
As catalytic reactions are important in fuel cells, the researchers are excited about the prospect of their research being used to generate the next generation of environmentally friendly power.