How can we engineer materials that are stronger and lighter? What about new materials for extreme conditions, such as in jet engines and spacecraft? The answer, says Fadi Abdeljawad, an associate professor of materials science and engineering in Lehigh University’s P.C. Rossin College of Engineering and Applied Science, might be hidden in the infinitesimally tiny regions, or boundaries, where atoms in crystals come together.
Along with his collaborators at the U.S. Department of Energy’s Center for Integrated Nanotechnologies (CINT), Abdeljawad is uncovering how those tiny boundaries have such an enormous impact on the characteristics of nanomaterials.
“Atoms come together to form nanocrystals, which are essentially structures about 1/10,000th the width of a human hair,” explains Abdeljawad. “Think of these crystals coming together like pieces of a puzzle, or as tiles on a kitchen floor. Billions of these nanocrystals stack on top of each other to form most engineering materials.”
According to the researchers, it is the regions where crystals meet that play an outsized role in determining how a material behaves. Recently, the team’s work was published in Nano Letters.
The article, “Triple Junction Segregation Dominates the Stability of Nanocrystalline Alloys,” explores how tiny features in nanomaterials, known as triple junctions, play a crucial role in maintaining the stability of these materials under high temperatures.
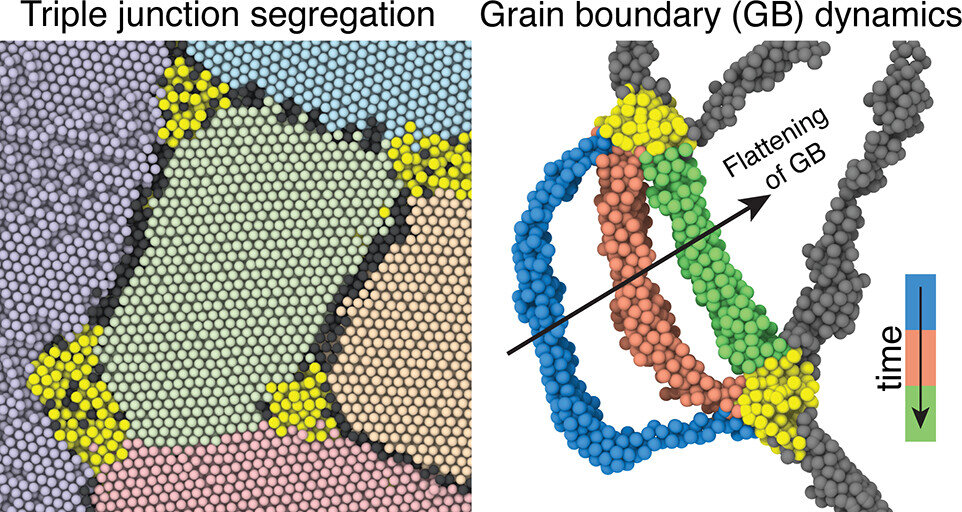
Gold in the corners
Nanocrystalline materials have an extremely fine structure, made up of many tiny crystals. This tiny crystal size can make the material stronger. However, keeping these crystals small and stable over time is challenging because they tend to grow, which can weaken the material.
The researchers in this study discovered that the key to maintaining the stability of these materials at high temperatures lies in triple junctions, corners where three of these nanocrystals meet. Imagine the corners of three puzzle pieces coming together.
What the scientists found is that when certain atoms are added to form an alloy, they prefer to occupy sites at these triple junctions. This “chemical segregation” or gathering of atoms at triple junctions helps to keep the grains from growing, thereby preventing the material from losing its strength over time.
This specific study demonstrated that gold atoms carefully placed at triple junctions in a platinum nanomaterial allowed the material to remain stable in conditions of high temperatures.
“By understanding this process,” says Abdeljawad, “scientists can design better nanocrystalline alloys. They can choose specific elements that will go to the triple junctions and stabilize the material. This is particularly important for applications where strength and durability at elevated temperatures are key, such as in the aerospace and energy industries.”
Drawing on the power of teamwork
Abdeljawad, a computational materials scientist at Lehigh, performed large-scale computational studies that predicted these results. To validate the models, the computational team partnered with the Center for Integrated Nanotechnologies (CINT). CINT provides advanced tools and expertise for nanoscale research, enabling cutting-edge studies in materials science, nanofabrication, and nanophotonics for scientific and technological advancements.
“This is an outstanding example of collaborative science,” says Dr. Brad Boyce, a senior scientist at CINT and a co-author on this study. “Our ideas for how to engineer novel materials by tailoring features at the nanoscale are maturing as a result of the ability to simulate the complex arrangement of atoms that make up these materials.”