Everyone knows it from their childhood days in the sandpit: sand is filtered using a sieve. Larger stones get stuck, while fine sand can fall through the sieve. The same principle is used when making coffee: water and aromatic substances can pass through unhindered, while the fine-grained coffee powder sticks to the coffee filter.
However, filtering purely by size is no longer sufficient for cleaning or separating chemical substances—e.g. colorants in water or various charged atoms, so-called ions. More sophisticated methods are required, for example, to separate different ions of similar size from each other.
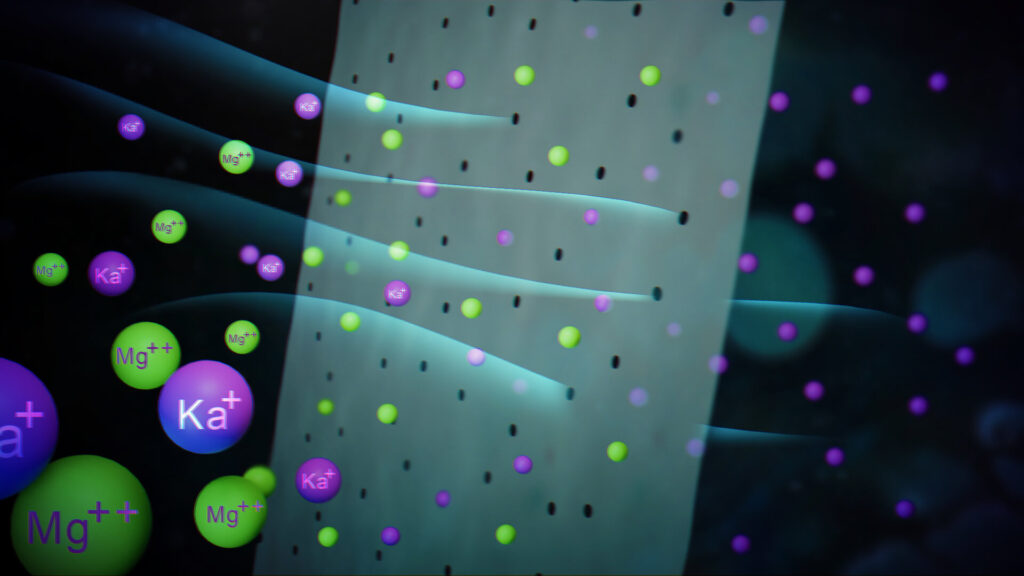
In biological systems, cell membranes can already carry out such separation processes by allowing additional chemical processes to take place in the thin “pores” of the sieve. In artificially produced sieves, however, this is still a major challenge.
Scientists led by group leader Christopher Synatschke in the department “Synthesis of Macromolecules,” headed by director Tanja Weil, have now succeeded in producing a membrane just 20 nanometers thick that can efficiently separate different types of ions or even a mixture of dyes.
The membrane consists of a material that is very similar to one produced by mussels: polydopamine. Using an electropolymerization process, the polydopamine membrane can be produced in such a way that it has sub-nanometer-sized channels, i.e. sieve pores.
Like a biological cell membrane, these sieve pores have a special surface chemistry. This makes it possible, for example, to separate monovalent ions (such as singly charged sodium) and divalent ions (such as doubly charged magnesium) despite their similar size.
“Such easily producible membranes are of great interest in applications,” says Synatschke. “This makes it possible, for example, to produce more efficient filters for water, e.g. for industrial waste.”
The researchers have compared the efficiency of their membrane with others. Here, they were able to achieve a remarkable selective separation between monovalent ions and larger species—more efficient than any other membrane produced to date.
The researchers hope that new applications can be developed from the new polydopamine membranes—because the material is not only environmentally friendly and biocompatible, but also particularly easy to chemically customize.
The study is published in the journal Advanced Materials.