New technology, which is in the early stages of development, has the potential to significantly enhance the effectiveness and reduce the side effects of drugs and vaccines.
The work is published in the journal Nature Chemistry.
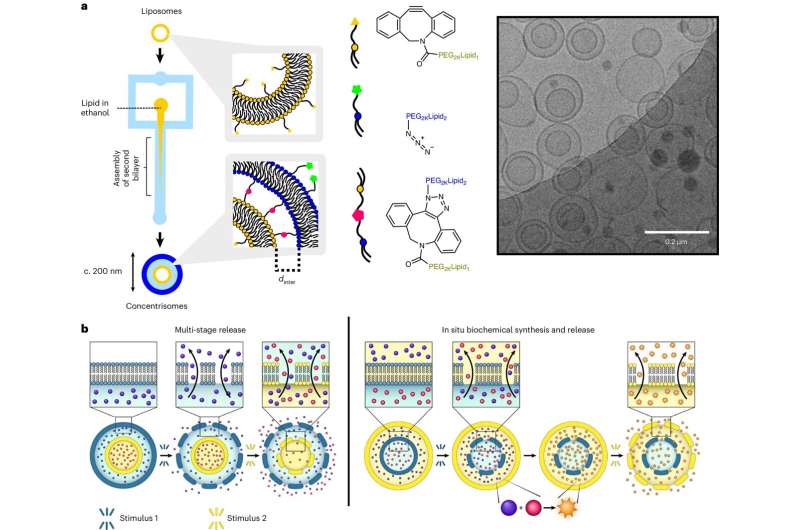
Drugs and vaccines can be delivered to the body using tiny packages called nanoparticles. The structures of these delivery particles are surprisingly simple, typically consisting of basic spherical clusters in which the drug or vaccine is held, before being released into the body. However, it is thought that more intricate architectures might provide more sophisticated properties, enhancing the efficacy and duration of therapeutics.
To address this, researchers at Imperial College London developed a new technology that lets them create compartments within compartments on the nanoscale. These compartments can mean timing drug release for maximum effect at the site it’s most needed. Timed release can make drugs and vaccines better targeted to specific sites of the body, which could increase their efficacy and reduce potential side effects.
Lead researcher Dr. Yuval Elani, of Imperial College London’s Department of Chemical Engineering, said, “Much like Russian dolls, our technology allows us to form particle-in-particle structures, with the ability to control all features of the particles, including the drug or vaccine encapsulated within each one. With further research to study how these nanoparticles interact with live organisms, this advancement holds significant potential for revolutionizing both therapeutics, such as chemotherapies, and vaccines.”
Inspired by nature’s cells
The researchers drew inspiration from the structural complexity of living cells. Dr. Elani said, “Just as the structural complexity of animal cells makes them capable of sophisticated functions, compartmentalized nanoparticles can be tweaked to exhibit more advanced features too.”
The designer nanoparticles are capable of multi-stage release, which allows one or two separate drugs to be delivered and released from the particle in several discrete bursts following one administration. This could replace the need for two or three separate vaccine shots spaced days apart.
They can also simultaneously release two different drugs, which could be crucial for combination therapies or adjuvant vaccine systems, where one molecule stimulates the immune response while another acts as the therapeutic agent itself.
The nanoparticles could also be engineered to create new drugs inside itself, triggered by encountering stimuli such a specific disease. This approach could enhance the therapeutic potential of the drug and reduce side effects, leading to better therapies and opening new avenues for patient treatment and care.
While the initial findings to demonstrate the feasibility of the technology are promising, the researchers say that it is currently at the proof-of-concept stage and has not yet been tested in an organism. Further research and development are needed to translate these findings into practical applications, including the testing and validating the system with actual drugs and vaccines in animal models to fully assess its efficacy and safety for medical use.
First author Dr. Colin Pilkington, from Imperial’s Department of Chemical Engineering, said, “There is a lot of buzz around lipid nanoparticle-based solutions to drug delivery. We hope this work will inspire others to consider more structurally diverse nanoparticle architectures, and that some of the ideas we have explored prove useful to the wider scientific community.”

